NOx refers to nitrogen oxides, and SOx refers to sulfur oxides, which are produced from the combustion of hydrocarbon fuels. These air pollutants negatively impact the environment by contributing to smog and acid rain, which damage ecosystems and contribute to climate warming. For living organisms, NOx and SOx are harmful as they can irritate the respiratory tract, cause breathing problems, and worsen cardiovascular health. Long-term exposure to high concentrations of these compounds increases the risk of respiratory and cardiovascular diseases.
Although not all NOx and SOx are directly classified as greenhouse gases, they can undergo chemical reactions in the atmosphere that lead to the formation of compounds with greenhouse properties.
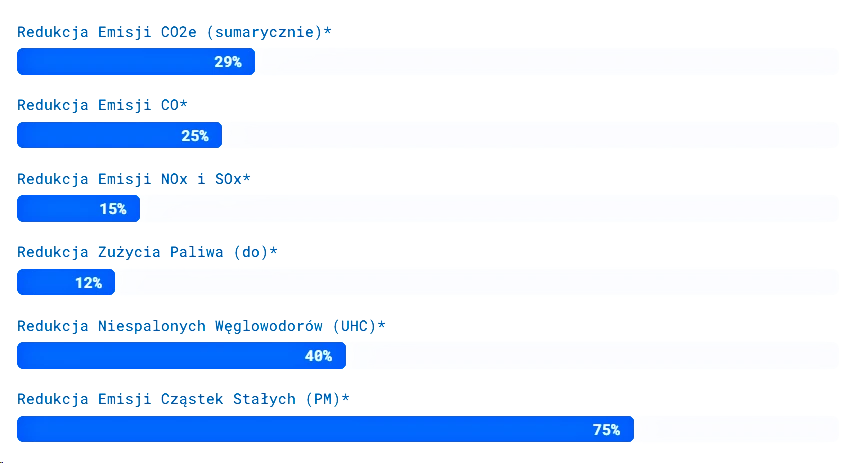
Therefore, reducing emissions of the entire NOx group, including N₂O, is a significant goal in reducing emissions from internal combustion engines.
Here are examples of such reactions:
1. Reactions of NOx with other compounds:
- NOx + hydroxyl radicals (OH) → HNO₃ (nitric acid) – contributes to the formation of ozone (O₃), which is a greenhouse gas.
- NOx + hydrocarbons → nitrogen-containing organic compounds – some of these have greenhouse properties.
2. Reactions of SOx with other compounds:
- SOx + moisture → H₂SO₄ (sulfuric acid) – contributes to the formation of aerosols, which can have greenhouse properties.
- SOx + ammonia (NH₃) → (NH₄)₂SO₄ (ammonium sulfate) – forms particulate matter that can affect the atmospheric radiative balance.
Thus, while NOx and SOx are not directly classified as greenhouse gases, they can indirectly contribute to the greenhouse effect through complex chemical reactions in the atmosphere.





Recent Comments